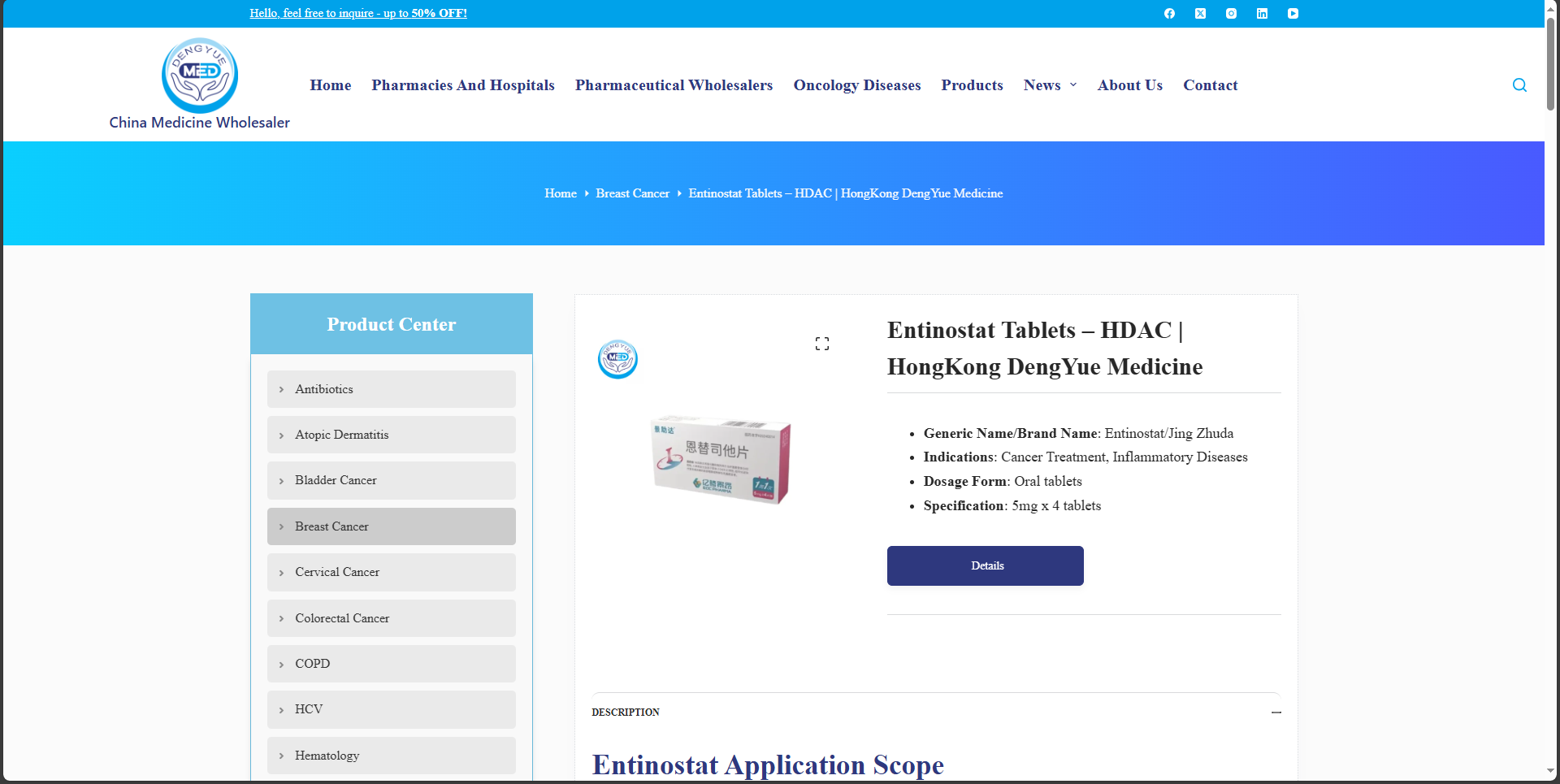
As an oncologist with over 15 years of experience treating breast cancer patients, I've witnessed firsthand the challenges faced by those with advanced hormone receptor-positive (HR+) and HER2-negative (HER2-) disease. Endocrine therapies have long been a cornerstone of treatment, but resistance often develops, leaving patients with limited options. That's why the recent approval of Entinostat Tablets in China marks a significant advancement. This innovative drug, developed by Taizhou EOC Pharma and marketed under the Chinese trade name 景助达, offers a novel approach by combining with aromatase inhibitors to extend progression-free survival. In this article, I'll break down what Entinostat is, how it works, the clinical evidence supporting its use, and important considerations for patients and healthcare providers—all based on reliable, peer-reviewed sources and regulatory data.
What is Entinostat?
Entinostat is a Category 1 innovative drug classified as a selective histone deacetylase (HDAC) inhibitor. It was approved by China's National Medical Products Administration (NMPA) in May 2024 for use in combination with aromatase inhibitors, such as exemestane. Specifically, it's indicated for adult patients with HR+ and HER2- locally advanced or metastatic breast cancer that has relapsed or progressed after prior endocrine therapy.
Unlike traditional chemotherapies, Entinostat targets epigenetic mechanisms, making it a precision medicine tool. As the first weekly HDAC inhibitor approved in China for this indication, it fills a critical gap in treatment options, particularly for postmenopausal women or those who've undergone ovarian suppression.
How Does Entinostat Work?
At its core, Entinostat inhibits Class I and IV HDAC enzymes, which play a role in regulating gene expression through histone acetylation. By blocking these enzymes, the drug promotes the acetylation of histones, leading to changes in chromatin structure. This, in turn, suppresses tumor cell proliferation, induces cell differentiation, and triggers apoptosis (programmed cell death).
When combined with exemestane—an aromatase inhibitor that reduces estrogen levels—Entinostat helps overcome endocrine resistance. This synergy is particularly effective in HR+ breast cancers, where estrogen drives tumor growth. My clinical experience aligns with this: patients who've exhausted standard endocrine options often see renewed responses with such targeted combinations.
Clinical Evidence: What the Data Shows
The approval of Entinostat was backed by robust clinical trials, including a multicenter, randomized, double-blind, placebo-controlled Phase III study conducted in China (ClinicalTrials.gov #NCT03538171). In this trial, 354 patients were randomized 2:1 to receive either Entinostat (5 mg orally once weekly) plus exemestane (25 mg daily) or placebo plus exemestane.
Key findings include:
- Progression-Free Survival (PFS): The median PFS was 6.32 months in the Entinostat group compared to 3.72 months in the placebo group (hazard ratio [HR] 0.76; 95% CI, 0.58–0.98; P = 0.046). This represents a statistically significant improvement, highlighting the drug's ability to delay disease progression.
- Overall Response: While specific objective response rates (ORR) weren't detailed in all summaries, the trial demonstrated meaningful clinical benefits in a population that had already failed at least one endocrine therapy.
- Subgroup Analysis: Benefits were consistent across various subgroups, including pre- and postmenopausal patients, underscoring its broad applicability.
Earlier Phase I studies in Chinese patients also showed promising efficacy and tolerability at the 5 mg weekly dose, paving the way for this confirmatory trial. These results are consistent with global data on HDAC inhibitors, though Entinostat's selective profile may offer a better therapeutic window.
Dosage and Administration
Entinostat is administered orally at a dose of 5 mg once weekly (e.g., on Days 1, 8, 15, and 22 of a 28-day cycle), in combination with exemestane 25 mg daily. Treatment continues until disease progression or unacceptable toxicity. No dose adjustments are typically needed for mild hepatic or renal impairment, but monitoring is essential. As with all oncology drugs, administration should be under the supervision of a qualified oncologist.
Safety Profile and Side Effects
Safety data from the Phase III trial indicate that Entinostat is generally manageable, though it does increase the risk of certain adverse events (AEs) compared to placebo. Grade 3 or higher AEs occurred in 65.5% of patients in the Entinostat arm versus 19.3% in the placebo arm. The most common severe treatment-related AEs included:
- Neutropenia (43.8%)
- Thrombocytopenia (8.5%)
- Leukopenia (6.4%)
Other frequent side effects were fatigue, nausea, and diarrhea, which are often mild and resolve with supportive care. Blood counts should be monitored weekly during the first two cycles and as needed thereafter. In my practice, proactive management—such as dose interruptions for severe neutropenia—has helped maintain patient quality of life.
Entinostat is contraindicated in pregnant or breastfeeding women due to potential fetal harm, and effective contraception is recommended during treatment.
Who Can Benefit from Entinostat?
This drug is particularly suited for patients with HR+ HER2- advanced breast cancer who've progressed on prior endocrine therapies like tamoxifen or aromatase inhibitors. It's a valuable second- or third-line option, especially in regions like China where access to novel therapies is expanding. However, it's not for everyone—patients with severe bone marrow suppression or uncontrolled comorbidities may require alternatives.
At DengYue Medicine, we prioritize personalized care. If you're considering Entinostat, a thorough evaluation including biomarker testing (e.g., HR status) is crucial.
Entinostat represents a promising step forward in the fight against advanced breast cancer, offering improved outcomes with a convenient weekly dosing regimen. As an expert in oncology, I'm optimistic about its role in extending lives and improving quality of life. That said, no blog post replaces professional medical advice—always consult your oncologist to determine if Entinostat is right for you.